- Home
- New Kyurizukai
- Study of Clathrate Hydrates
from Keio's Faculty of Science and Technology
Study of Clathrate Hydrates
from Keio's Faculty of Science and Technology
Crystal engineering for energy
and environmental technologies.
Logical thinking is essential for persuasive research. I’d like to address vis-à-vis what I feel is interesting.
Dr. Ohmura has been tackling research on clathrate hydrates ever since he was an undergraduate. While he continues to pursue his career as a researcher, he also devotes his energies to fostering the next generation. When it comes to thermodynamics, his specialty, he smiles and jokingly says, “For students, this must be one of the toughest and ‘worst’ subjects to deal with.” Contrary to his self criticism, his students favorably comment on Dr. Ohmura’s lectures as being easy to understand. Some say that the study of physics has become interesting thanks to Dr. Ohmura.
Ryo Ohmura
Dr. Ohmura’s specialties are thermodynamics and physical chemistry. His current research projects are physical chemistry of clathrate hydrates and the development of energy- and environment-related technologies. His activities range widely from basic research to applied research for practical application. After acquisition of a doctor’s degree (engineering) in 2000, he visited France to participate in a hydrate research project. For four years from 2002, he served as a research scientist at the National Institute of Advanced Industrial Science and Technology (AIST). In 2006, he arrived at his post as an assistant professor of Keio University Faculty of Science and Technology, and then he was promoted to his current post as an associate professor in 2009.
Introducing Researchers
Featured in this issue is Associate Professor Ryo Ohmura, who tackles new technological development in the energy field by utilizing clathrate hydrates.
Clathrate hydrates useful for natural gas storage and CO2 extraction
Effective utilization of materials that are similar to but are not ice
Clathrate hydrates have a cage-like structure made of water molecules in which a substance other than water has been sealed and crystallized. Substances that can be sealed in the cage structure include hydrophobic gases such as methane, nitrogen, and carbon dioxide (CO2). While clathrate hydration is a focus of attention as an effective gas solidification technology, many mysteries still remain as to its principles and mechanism. Keio University’s Faculty of Science and Technology boasts one of the world-leading achievements in the study of clathrate hydrate application technology. Associate Professor Ryo Ohmura, the person who spearheads this research activity, explained what clathrate hydrates are like.
Water molecules form a cage in which a guest substance stays.
Dr. Ohmura explains the characteristics of its structure: “Clathrate hydrate is a crystal of orderly arranged water molecules. Though it resembles ice in appearance, it differs from ice in the way water molecules are arranged. Unlike ice, in which water molecules are closely arranged leaving no space between them, clathrate hydrate features a polyhedron cage structure made of water molecules. The cage structure takes in a non-water guest substance and is then crystallized.”
Imagine the traditional “Kagomekagome” game of Japanese children and it will easily explain the clathrate hydrate structure. In the center of a circle is a tagger bending his or her body with eyes closed. The surrounding children, tanking each other’s hands, are compared to water molecules. The tagger is not left out of the game but is surrounded at a distance from the others. The tagger can neither get hand-in-hand with the others nor is allowed to get out of the circle. The guest substance in clathrate hydrate is something like the tagger in the “Kagome-kagome” game.
It solidifies a hydrophobic gas and enables its efficient storage and transport
Clathrate hydrates with such characteristics also exist in the natural world. Methane hydrate, recently in focus of attention as a promising natural resource, is a kind of clathrate hydrate with its guest substance being methane molecules.
Similar to ice, methane hydrate is attracting attention as a medium capable of storing methane gas at a high density. Mr. Ohmura addresses the research project of taking advantage of such excellent storage capacity of clathrate hydrates for energy utilization of natural gas.
“Methane hydrate is a crystal containing water and methane molecules at a ratio of 46 to 8. Methane hydrate’s storage capacity is 170 times greater in volume than that of methane gas. I came up with the idea of sealing natural gas as a guest substance. This marked the beginning of my study of natural gas hydrates. This research endeavor has come to the stage just before commercialization or engineering practice. The foremost merit of natural gas hydration is its ease of temperature management. Normally, natural gas is stored and transported as liquefied natural gas (LNG) after being cooled down to –162°C or less. By making it into the hydrate form, natural gas can be compressed to 1/170 in volume at a temperature of only –20°C. Though the volume of natural gas hydrate increases 3.5 times greater than that of LNG, it eliminates extra maintenance and management costs associated with cooling it to the cryogenic temperature of –162°C or less, making methane hydration highly promising as an alternative technology.”
Since hydration enables a guest substance to be stored at the molecular level, its application for the storage of hard-to-store or dangerous substances is being examined positively.
“Ozone (O3) that exhibits superb bactericidal actions is a very unstable substance. In principle, ozone cannot be stored as is since its molecules react to each other and turn into more stable oxygen (2O3 → 3O2). Once hydrated, howe ver, it can b e s eparated and preserved in the molecular form, thus enabling storage over a period of long time,” he remarks.
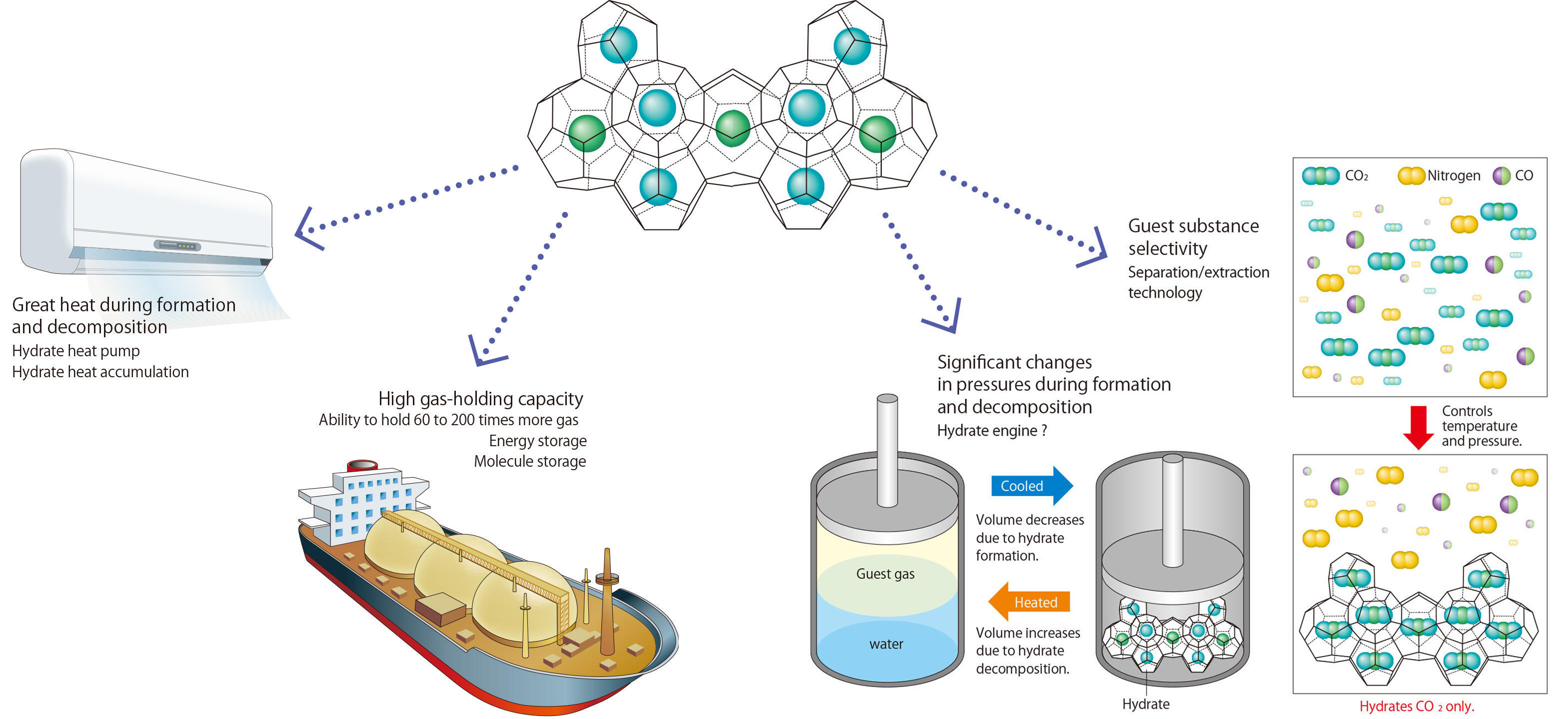
Fig.1 Application technologies that make the most of clathrate hydrate’s physical characteristics
Highly efficient use and handling of clathrate hydrates becomes possible by incorporating their superb physical characteristics (greater gas storage capacity and greater decomposition heat) peculiar to clathrate hydrates into existing technologies and systems. Examples of application include natural gas storage and transport technology, carbon dioxide separation and extraction technology, and application to high-efficiency heat pumps. As future possibilities, innovative technologies, such as a hydrate engine, are also being conceived.
Can extract a specified substance and is promising as a CO2 removal technology.
Clathrate hydrate cannot be formed by simply mixing water and a guest substance together. The formation of clathrate hydrate requires conditions of low temperature and high pressure. However, such conditions vary according to the guest substance in question. For example, at the temperature of 0°C, methane can be hydrated under 26 atmospheres (approx. 2.6 MPa), while carbon dioxide (CO2) and nitrogen require 12 atmospheres and over 150 atmospheres, respectively.
“By taking advantage of such differences in hydrate-forming conditions, it is possible to hydrate particular guest substances selectively. Attracting attention as an application of this is the technology for CO2 separation and removal from exhaust gas emitted by thermal power plants. It is an attempt to selectively solidify CO2 only. In this process, exhaust gas, which consists of carbon dioxide and nitrogen, is given an optimum condition for hydration by having it react with water. Currently, the amine process* is used commonly as a CO2 removal technology, but it involves the necessity to handle amine, a dangerous substance. Contrary to the amine process, the removal process, based on hydration of CO2, uses water only, making it a highly promising process as an alternative to the amine process.”
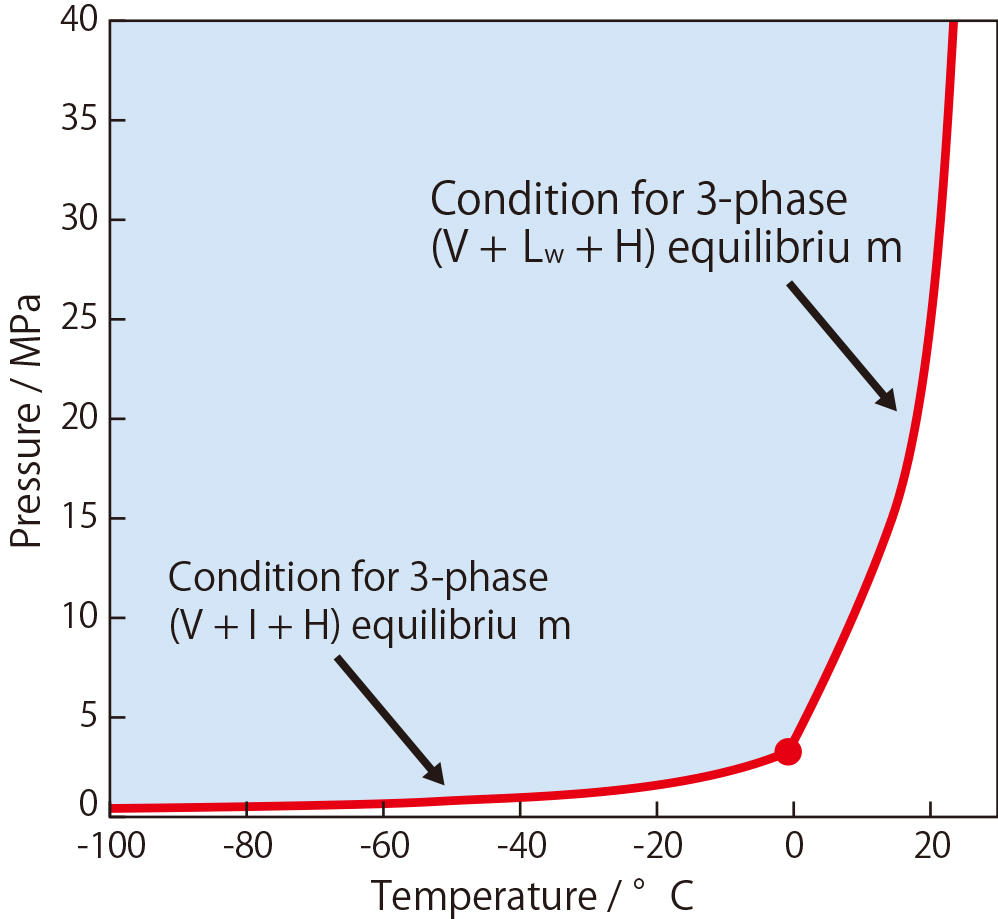
Fig.2 Conditions required for methane hydrate formation
Methane hydrate can be formed only when methane, water molecules, and temperature and pressure conditions become complete. The graph on the right shows the relationship between temperatures and pressures. When the temperature is low, the required pressure is low accordingly. But a higher pressure is required as the temperature rises. However, the low temperature and pressure required for control remain within a controllable range. Thus, ease of handling is another characteristic of methane hydrate. These physical characteristics can be seen commonly among all clathrate hydrates.
Also expected as a highly efficient thermal storage medium substituting for ice thermal storage
As clathrate hydrate is formed, its water molecules turn into a solid ice-like state, which can be controlled at a temperature range of between 5°C and 15°C. The creation of an efficient thermal energy storage medium for cooling can be expected as an application of this physical characteristic.
“Currently, most large buildings employ an ice thermal-energy storage system for their air-conditioning, which uses nighttime power to make ice and uses its cold air as the air-conditioning thermal source. From an efficiency viewpoint, however, it is pointed out that the thermal source of 0°C is too low since a thermal source temperature of approximately 10°C is optimum for producing cool air of 15°C needed to maintain the room temperature of 28°C. In fact, as much as 40% power conservation can be expected if the thermal source temperature is raised to 10°C. With hydrate thermal storage, it is possible to create a thermal s ource of approximately 10°C by controlling the guest substance and pressure – a thermal storage system far surpassing the efficiency of conventional ice thermal storage systems.”
As Dr. Ohmura puts it, however, hydrate thermal-energy storage is not without problems. To realize practical application of clathrate hydrates, not only must the safety of guest substances be verified beforehand, but also the technology’s principles and mechanism must be brought to light, the knowledge of which should be shared by many researchers and opinion leaders.
“As the stage of practical application of this technology approaches, now is the time for us to devote ourselves to basic studies. As a person in the academic world and as a leading researcher in this field, I think we now have to approach the clathrate hydrate phenomenon from a broader perspective, not biased for a particular engineering field,” he concluded.
Studies of clathrate hydrates are steadily in progress.
Interview
Listening to what Associate Professor Ryo Ohmura has to say
Father’s advice guided me to choose the science and technology course at university.
What was the impetus for you to choose the science and technology course at a university?
I’m now a teacher of mechanical engineering at Keio’s Faculty of Science and Technology. It may surprise you to say that mathematics and physics were my weakest points as a high school student. My favorites were social-study subjects, such as politics, economics, ethics, and history.
Why did a liberal arts-oriented boy like me choose to study science and technology at a university? It was because of my father’s advice. When I was pondering which subjects to choose at university, my father said, “A university can offer great opportunities because you can meet good teachers who are specialists in their respective science and technology fields. Why don’t you learn your weak subjects from them?” He also added, “You can study liberal arts subjects on your own, but it’s difficult to learn scientific subjects alone.” His remark touched my heart, which made me feel persuaded. Fortunately, my scores in entrance exam mathematics and physics tests were passable, so I decided to choose the science and technology course and went on to the Department of Mechanical Engineering of Keio University’s Faculty of Science and Technology.
How did you manage your weak points – scientific subjects?
Although my perception of mathematics as being my weak point remained unchanged, my impression of physics totally changed immediately after admission to Keio. My teachers in charge of lectures on mechanics and thermodynamics were so professional that I was truly impressed by the fact that those teachers with thorough knowledge could make lectures so exciting.

At the university, I could learn everything about physical phenomena and machine functions through lectures and experiments – why is it so and why does it move like that? – from their principles through to mechanisms. Since every one of the teachers had involvement with the industrial world in one way or other, they had practical knowledge of how mechanics and thermodynamics could be useful for various applications. Given such backgrounds, their lectures were very persuasive and they could respond to students’ questions with substantial answers supported by their own practical experiences. These lectures were totally different from my high school physic class where I studied while asking myself “What on earth would this study be useful for?”
An encounter with Prof. Sloan motivated me to begin serious research into clathrate hydrates.
What was the impetus for you to begin studying clathrate hydrates?
It was when I joined Prof. Yasuhiko Mori’s laboratory as a senior. In those days, Dr. Mori focused on research into heat transfer, one of its themes being clathrate hydrates. Although I found clathrate hydrate phenomena intriguing back in those days, I didn’t give it much further thought. But my perception of clathrate hydrates changed dramatically when I had an opportunity to meet Prof. E. D. Sloan Jr. of the Colorado School of Mines.
Prof. Sloan is the leading figure in the field of clathrate hydrate study with outstanding achievements in engineering. When I was studying in the master’s program, a special course was held in our School of Science and Technology inviting Prof. Sloan. Though the special course lasted only for two months, it was a truly inspiring experience for me as it allowed me to acquire systematic knowledge from Prof. Sloan, whose research activity had focused on clathrate hydrates for years.
I must admit that the clathrate hydrate research I had been engaged in up until then was merely at the stage of a development from the heat transfer engineering. The encounter with Prof. Sloan turned out to be a breakthrough as it also allowed me to learn in depth about basic physical properties. Surprises and possibilities I could notice by learning vis-à-vis Mr. Sloan, not through theses, had a great impact on the future course of my research activities.
When I was thinking of continuing my clathrate hydrate research pursuit after acquisition of a doctor’s degree, I happened to know that the National Institute of Advanced Industrial Science and Technology (AIST) was looking for young researchers in the field of hydrates. I was quick to apply for a position there. Many of the senior researchers with whom I worked in the AIST project were specialists in applied physics; hence the mainstream of their research style was to focus on the pursuit of physical properties. As a researcher from the mechanical engineering field, I still remember that the approach from applied physics was fresh and rewarding. I belonged to the AIST for four years, during which period I experienced practical aspects of engineering science – the field where I could engage in fundamentals of applied physics while simultaneously observing research targets from the engineering perspective. It was a truly valuable experience as it concerns my current research activity.
After leaving AIST, you returned to Keio. Was there any special reason for doing so?

Since my student days, I had thought I’m of the type who encourage others and draw out their potential abilities, which I became strongly aware of during my service with the AIST.
While AIST researchers sometimes work with their subordinates and part-timers, most of them normally devote themselves to their own research tasks. But I came to feel more motivation in thinking and working with students and fostering students who are willing to contribute to society and universities, and to shoulder the future for all. So I chose a career as university teacher, seizing an opportunity of an offer for a post of assistant professor. In my view, I’m a type of teacher who develops students’ potentials by encouraging them while maintaining sternness. I know I’m viewed as a stern teacher among students, but think it’s okay to some extent.
I divert myself by jogging to renew my energy to draw out students’ potentials.
Aside from your research work, what are you interested in, or what are your pursuits?
I like to move my body. Jogging is a longtime pursuit. These days, I make it a rule to run a distance of about 16 kilometers daily, or one hour and 40 minutes. Since my home is in the Tsunashima area, my regular route is heading toward Shin-Yokohama along the Tsurumi River to the Nissan Stadium and back. When I have no afternoon engagement, I sometimes leave the campus (Hiyoshi) and go to the Tama River, enjoying a round trip to and from Denenchofu along the riverside promenade. It’s almost a habit rather than a hobby. While running, I can put my thinking in order, whereas being able to refresh myself when I have a problem and waver in my judgment.
Occasionally I bench press and do other forms of muscle training. Our lab has a bench for bench press, with which we break a sweat two times a week jointly with members of the Mori lab. In bench press exercise, we compete with others not by the weight (more correctly, the mass) one can raise, but by an index obtained by dividing the mass with one’s body weight. We nicknamed this index the “Mori value.” Most lab members are striving to achieve 1 Mori value, the equivalent of one’s body weight. The maximum mass I can lift is 95kg. Given my body weight of 54kg, my Mori value comes to 1.76.
Other than physical exercise, I enjoy veranda gardening. From summer to autumn, cultivation of herbs is in season. I’m also cherishing young lilac plants which were given as a souvenir when I visited Hokkaido. Tokyo’s summer heat was so terrible for the lilac plants that I moved them to a cooler place in the shade. It’s a small consideration but is indispensable in order to enjoy the flowers as a messenger that tells the arrival of spring.
Please tell us what you are doing consciously as a teacher to draw out and develop students’ potentials.
“Always be logical,” I would say. I’m convinced that the ability to think logically is essential to make the best use of oneself in a given organization or society. Point 1: I think that sometimes it’s important to respect the rule prevalent in a given environment. It’s important because if you go out into the world, you have to prepare yourself to encounter realities beyond your control. Point 2: Make it a rule to think about things by putting yourself in the shoes of others. One tends to be self-assertive when one has a strong message to deliver to others. But it’s wise to restrain yourself. This holds true when it comes to research presentations. The greater your research achievement is, the less it can be communicated to the audience because it’s too great to be understood by others. If that’s the case, you should pull back one step and try to think how, as a person before discovery of that great achievement, you might have reacted to it. I’m trying to experience and prepare such environments through research activities and academic presentations.
From a mechanical engineering point of view, I’d like my students to acquire the practical application ability based on the laws of physics, with which they can judge things correctly. For example, when it comes to a diet-related topic, such as “Whether it’s possible to remain slim even if you eat as much as you like,” I’d like them to view it from laws of physics and say “It’s against the law of energy conservation.” If in daily lives they become conscious of the laws of physics that govern the real world, they would be able to find physics interesting. I know that mechanics and thermodynamics are extremely hard to understand, but it’s true that these sciences are more useful than any other sciences once you have acquired such knowledge. I’d like to offer lectures in such a way as to make my students become aware that these sciences are indispensable for establishing the structure and systems of society.
Just a word from . . .

A student : In a single word, Dr. Ohmura is a man of knowledge. Not to mention his lectures, he has a wide range of topics to talk about, from history and culture as well as cooking, food ingredients, and wine. We are totally brought into his world before we know. Whenever we have a drinking party at our lab, Dr. Ohmura is kind enough to serve us tasty wine and cheese. His explanation of the wine’s origin and the winery’s characteristics is truly intriguing. We always look forward to listening to him. He is also good at sports. I’m sure his physical strength is greater than ours!
(Reporter & and text writer: Kaoru Watanabe)


