- Home
- New Kyurizukai
- "Man-Made Proteins" from Keio University's Faculty of Science and Technology
"Man-Made Proteins" from Keio University's Faculty of Science and Technology Invention and creation through researching self-assembling proteins
Finding the fun in the trials and tribulations of research
Professor Kawakami has conducted research in a variety of specialties dating back to his time at a Japanese college of technology, or “kosen.” From strategies on how to survive as a researcher, experience on how to get out of tight spots, and an understanding of how to produce results, Kawakami’s skills are on constant display in his work at Keio University.
Norifumi Kawakami
Specializations in protein science, enzyme engineering, and the bio-metal science . 2004 graduate of the National Institute of Technology, Ube College (NITUC) with a degree in Chemical and Biological Engineering. In 2009, he completed the coursework for a Ph.D. at the Department of Biological Science, Graduate School of Science, Hiroshima University. Ph.D. in Science. After working as a postdoctoral researcher at the International Research Center for Materials Science and at the Department of Biological Science at Nagoya University, he became a project assistant professor at Keio University’s Department of Biosciences and Infomatics in the Faculty of Science and Technology in 2014, and a senior assistant professor in 2017, a position he still holds.
The Research
This issue features Senior Assistant Professor Norifumi Kawakami and his ongoing research about creating soccer ball-shaped protein nanoparticles.
Self-Assembling Soccer Ball-Shaped Protein Nanoparticles
Towards next generation ’s nanomaterials
Proteins: a fundamental building block of all living organisms. Norifumi Kawakami has harnessed the properties of proteins in his creation of soccer ball-shaped nanoparticles. These nanoparticles can be broken down and still return to their original shape, meaning that they are expected to have incredible utility as nanocapsules for delivering medications inside the human body.
Creating a name and legacy through a molecule
When Professor Kawakami was hired to his current position in April 2014, he set to work on a research project that would “create a molecule that’s never existed before in our world.” In explaining his motivation for this project, Kawakami says, “I’ve done research in so many different areas that you could say that there’s been no real consistency to my work. I realized that in order to survive as a researcher, I would need to make a name for myself, some achievement that would automatically be associated me, almost like a research ‘calling card.’” Because of Kawakami’s frequent encounters working with proteins in the past, he decided to use them as his focus when creating the new type of molecule. For his research “motif” he landed on the idea of a soccer ball. “When I was a student, I was fascinated by the beauty of fullerenes. A fullerene is a form of sixty carbon atoms arranged in a pattern like a soccer ball. (Fig. 1) I later learned that this soccer-ball shape occurs naturally in everything from plants to outer space, so I thought there might be something special about it and some reason why it forms so readily. This was my inspiration in wanting to design a soccer ball-shaped molecule.”

Figure 1: “Soccer ball” patterns appearing in various settings A fullerene composed of 60 carbon atoms (left), newly budding pincushion flowers (center), and Kronberger 61, a planetary nebula shaped like a soccer ball (right). Rightmost photo courtesy of the International Gemini Observatory/AURA
Making a soccer ball-shaped molecule using fusion proteins
So how exactly is it possible to take proteins and make them into a soccer ball-esque molecule? Kawakami’s chosen molecular design method was to use fusion proteins. Inside our bodies, proteins are at constant work, spontaneously and automatically collecting into complex structures. Around the year 2000, researchers began to investigate ways that they could use this property to manipulate proteins into man-made shapes.
Then, in 2014, an American team of researchers successfully constructed a polyhedral molecule by using fusion proteins which combined two types of proteins together. However, a problem emerged; the proteins could take multiple different polyhedral shapes. Mixed types in a product create a major roadblock to industrial applications. Kawakami saw this issue and began to explore whether there was a way of only producing soccer ball-shaped molecules.
“Soccer balls are essentially just polyhedrons made up of 12 pentagons and 20 hexagons, so my first thought was to figure out some sort of pattern that combined these shapes. The problem was that I had no idea how to arrange them together in a way that made sense and no clue how to make their edges line up.” However, as Kawakami stared down the blueprints for his molecule model, he realized something. “I was stuck on the idea that hexagons were essential to building a soccer ball, but then I realized that if you simply use lines to connect pentagons to each other at their vertices, inevitably you will end up with hexagons.” (Fig. 2)
Using Euler’s theorem on polyhedrons—(number of vertices) - (number of edges) + (number of faces) = 2—it follows that a polyhedron consisting of hexagons and pentagons must include 12 pentagons. In other words, it is impossible to create any shape other than that of a soccer ball if relying on a mechanism which connects the lines extending from pentagons’ vertices. “This is it!” Kawakami was confident he had found the solution.
The project researchers then designed a fusion protein (Fig. 3) and were able to confirm that the only types of molecules that should result from their experiments would be shaped like soccer balls. However, these particles were microscopic—only 22 nanometers in diameter (a nanometer is one billionth of a meter)—meaning that any tests to categorically determine whether the molecules were structured like soccer balls would require advanced techniques and equipment. Thanks to the help of the joint researchers on his team, Kawakami was able to conduct an analysis using cryo-electron microscopy (cryo-EM), a technique for which the inventors were awarded the 2017 Nobel Prize in Chemistry. Five years after the particles were first constructed, Kawakami was finally able to demonstrate conclusively that the molecules were, in fact, shaped like soccer balls.
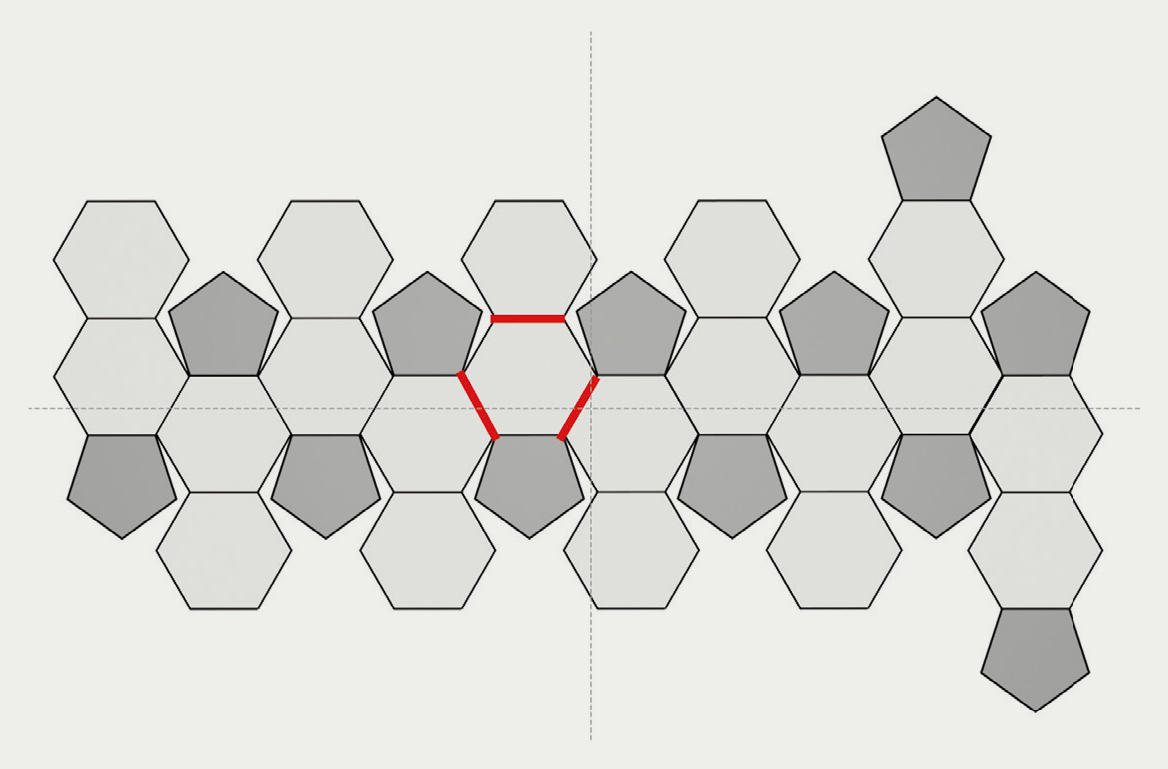
Fig. 2 The geometric net of a soccer ball Soccer balls are made up of 12 pentagons and 20 hexagons. The vertices of connected pentagons (red lines) naturally form hexagons.
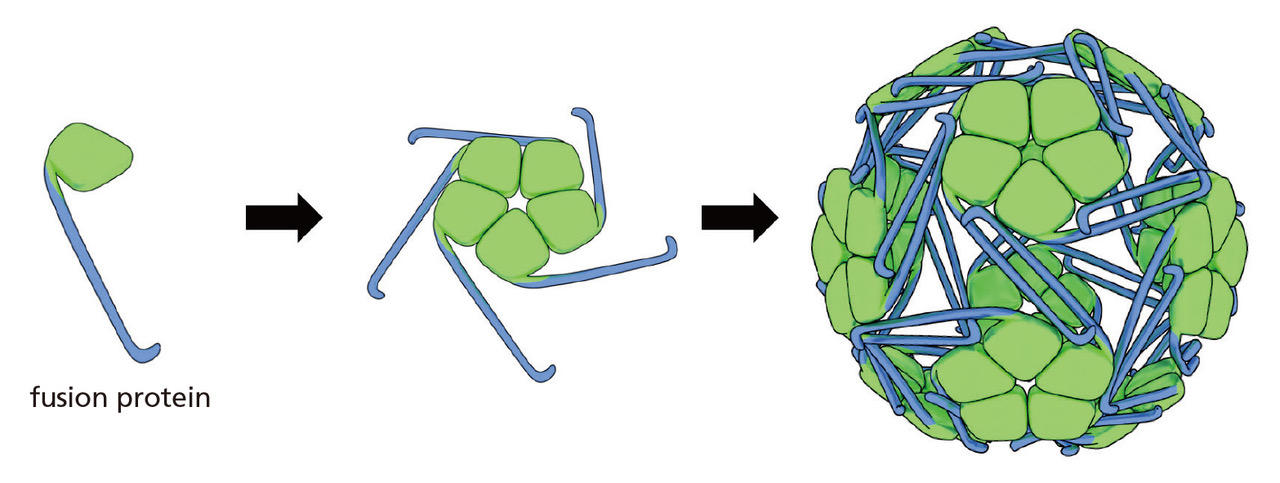
Fig. 3 Design concept for creating the soccer ball-shaped molecules. The scientists fused two types of natural proteins so that when 60 of them were collected, they would form the shape of a soccer ball. The green and blue proteins have properties that are attracted to each other. Furthermore, the blue proteins are hooked, meaning that when they latch to each other, they stabilize the molecule’s structure. In practice, the fusion proteins are created when their genes are introduced to bacteria from the large intestine (E. coli). The bacteria synthesize the proteins, which then spontaneously gather and form into the shape of soccer balls.

Fig. 4 The model (photo) and actual structure of the molecule as revealed through cryo-EM (right) Just as intended, the 60 individual proteins ended up forming into a soccer ball-shape. The photographed piece of the model shows how five proteins are amalgamated into a pentagonal piece. The final 60-molecule nanoparticle is a truncated icosahedral protein, which led to the name “TIP60.”

Potential applications for nanocapsules and nanomaterials
While Kawakami originally started this research so that he could have an identifiable accomplishment associated with his name, the response to his soccer ball-nanoparticles in academic papers and at conferences was enormous. Because the centers of the nanoparticles were hollow, it was possible to fill them with medicine, allowing them to be used as “nanocapsules” or for other futuristic applications.
It was at this point that Kawakami then invented a way for the nanoparticles to disassemble and reassemble themselves into their soccer ball-form. If a manufacturer adds a substance when the particles are reassembling into their original shape, this substance will be trapped inside the ball.
In a more recent development, one of the students working on the research project figured out how to produce large quantities of the soccer ball nanoparticles at a low cost. The team is also working to produce a soft, gel-like material where the nanoparticles form large structures that store water around them. This mechanism would make it so that when the gel is activated, the soccer-ball particles will “collapse” allowing whatever they carry inside to be released.
When asked about his thoughts and aspirations for the future of nanoparticles, Kawakami says, “My goal is to have people all over the world use these soccer ball nanoparticles. That way, when other researchers see how we were able to package other substances inside them, turn them into a gel, and find different uses for them, they might look back on our work making the soccer balls when exploring materials and chemicals of their own. That would feel incredible.”
Interview
The Interview: Senior Assistant Professor Norifumi Kawakami
Hating studying back in middle school
Did you like studying when you were younger?
Not even a little. When I was in elementary school, I could get good grades on tests without studying at all. By my second year in middle school though, I couldn’t follow along by just listening during class anymore, especially for English. Even if I had questions about how a preposition like “for” was being used in a sentence, no one would teach me. As time went on, the things I didn’t understand just kept piling up and I ended up hating it all.
At home, I would pretend to study, but in reality, I was reading manga and playing video games. Looking back at it, I must have been quite the handful.
Learning the joys of learning when attending a Japanese college of technology
After middle school, you ended up going straight to a “college of technology,” or “kosen,” right? (For our international readers, kosen refer to five-year programs or engineering schools that middle school graduates can attend to get an associate degree.)
Honestly, my first preference was to continue my education at a high school, but one of my middle school teachers told me that my grades would make that difficult, so somewhere along the way I ended up choosing to go to a kosen. I didn’t really know what type of place it would be, but after I started attending, it ended up fitting me perfectly. There were a lot of atypical students, so I managed to fit in pretty easily. I was at home with them.
Also, the kosen teachers were like college professors. They each had their own specialties, and whenever they talked about things in their personal area of expertise, you could really tell that they had fun explaining what they were teaching us. Whenever I expressed confusion, they would take my questions seriously when answering. If there was no clear answer, they would suggest potential thought strategies about the topic so that I could get my head around everything. It was also a lot of fun watching the teachers interact with each other. I remember thinking “this is how science gets done, by people working like this.”
That was a big moment for me. Up through middle school, I always thought that “studying” meant memorizing the information in textbooks and then filling it out on answer sheets. But as I listened to my teachers’ conversations, I was able to come to terms with the idea that the things being taught might be useful. As I kept working to understand the things that interested me, my grades in those subject areas steadily increased.
Making plans with a mentor
Why did you decide to pursue research as a career?

Kosen are designed so that their main programs last for five years, but for students who are interested in pursuing a higher level of education, they also offer 2-year majors. A lot of kosen students end up entering the workforce after they’ve finished their core coursework, but I felt like if I had come this far, I might as well continue to do a major course.
During my time at the school, I studied ceramic water filtration and other water purification methods and was greatly influenced by my then-advisor, Dr. Shiro Kubuki (who now works as an associate professor at Tokyo Metropolitan University.) When I was unsure about what I should do after graduation, especially since I had realized how much I enjoyed research, he was the one who recommended that I go to graduate school.
At the time, I remember him nonchalantly saying that he would like to collaborate on a project together if I made it as a researcher, but it felt like a bit of a pipe dream to me back then. As it turns out, in addition to my soccer ball nanoparticle project, I’m also working on some research on biological evolution using Escherichia coli which seems like a good match for Dr. Kubuki, so I think that a joint research collaboration might be a very real possibility in the near future.
When was the first time you found research to be fun?
When I was studying under Dr. Kubuki, there was a lot of interest circulating about “photocatalysis.” A photocatalyst is a substance that exhibits a chemical reaction when exposed to light. A typical example would be titanium dioxide. The photocatalytic properties of titanium dioxide are used for air purification and antibacterial purposes.
At the time, I did not really understand how it all worked, but when I picked through enough keywords to get to “light can cause compounds to dissolve through catalytic action,” I managed to put together a hypothesis. My idea was that if titanium dioxide was placed in sugar water and exposed to light, the light would begin a catalytic action and break down the sugar, lowering the solution’s concentration. As it turned out, the experiment had the opposite result and the sugar concentration increased. Thinking that this might mean I had made a huge discovery and synthesized some sort of compound, I went to report to Dr. Kubuki, brimming with enthusiasm. He quickly disillusioned me by saying that all that had happened was some of the water evaporating.
Thinking about this now, the results were obvious, but it was my first time understanding how research could be exciting. You come up with an idea, test it, and even if the results of your experiment are not what you expected, you can find an explanation for what happened. I was exhilarated by the possibility that hypothesis-driven research could be this fun.
Survival skills for a postdoctoral
So, after that, you completed your program at Hiroshima University and started your postdoc at Nagoya University.
That’s right. In graduate school at Hiroshima University, I studied chrysanthemums and ascidian. After that, I was hired as a postdoctoral fellow at Nagoya University in the Laboratory of Bioinorganic Chemistry, which focuses on proteins. However, I spent all my time at Hiroshima University researching biological systems, so I had not been involved in chemistry research since I was in my kosen program. I didn’t even know where to start to get involved in a research topic. I decided to ask the other students what was the most difficult or interesting chemical reaction they knew about at the lab. If I was somehow able to produce a chemical reaction that everyone else thought was impossible, I figured it would benefit both the lab and my ability to survive as a researcher.
As luck would have it, we did manage to produce exactly such a chemical reaction. The only problem was that there was a German research lab which had submitted a research paper on the same topic at the same time. If our paper’s submission was even slightly later than theirs, the chemical reaction would be credited to the German researchers. If that happened, I feared that I might reach the end of the road in my research career.
Things to value as a researcher
And this research experience is what later led to your success in creating the soccer ball-shaped nanoparticles?
Well, actually, after I came to Keio, I was working on another molecular design project before the soccer-ball nanoparticles, but I just couldn’t get it right. It was one failure after another. I had a professor at Tohoku University analyze some of the molecules I had created so that I could learn about their structures. They told me that “it’s pretty much garbage” after their observations revealed that the particles were all a bunch of different sizes.

This happened after putting a little over a year of work into that project, so it was really discouraging. My contract at the time could only last up to three years. I remember thinking that I had finally reached the end of my time as a researcher, but as soon as I came to this conclusion, I realized that I might as well go out on a high and work on the molecular design I wanted to do. On the bullet train ride home from Tohoku University, I turned to one of my students and said enthusiastically, “We’re going to make a soccer-ball shaped molecule.” I’m not really the type to dwell on things. I think that’s one of the reasons I’ve been able to come this far. I have a strong desire for my research to be useful to other people, but when it comes down to it, I create what I want to because it brings me a sense of self satisfaction. For me, molecular design is like the feelings a person might get from working on a painting. While it is demanded that research be functional in our day and age, I also want to prioritize my personal interests and the internal inquisitiveness that research elicits.
Creating an inviting workspace for research
Since coming to Keio University, what is your impression of the atmosphere here and in your laboratory?
Compared to the national universities, I would say students are given a large degree of freedom. Students are very honest and upfront with the teachers, so it’s easy to talk with them, get an idea of their personalities, and provide guidance as necessary.
I’ve experienced quite a few different laboratories and the atmosphere in the lab can drastically influence the level of motivation everyone feels towards the research. This is why I want to prioritize making the workspace as inviting as possible. I make a point of talking to the students regularly as a part of my teaching philosophy.
Some words from Students…
● The reason I chose Professor Kawakami’s lab was because I found the undergraduate class he taught to be really interesting. I could really tell that he was working on his research because he enjoyed it. I’ve been involved with the soccer ball-nanoparticle project and I’m thinking that I would like to continue working in research in the future. The world of research isn’t just about finding the “right” thing to work on. I’m inspired that choosing the “interesting” thing can also lead to results. (2nd year doctoral student).
● I am working on the E. coli biological evolution research project. I chose this laboratory largely because of Professor Kawakami’s personality. He’s enthusiastic about answering any questions I have and keeps things light by joking around with us a lot of the time, so it’s really easy to approach him for advice. He’ll discuss things with us until we’re all satisfied with things, so I feel like I can be proactive in making progress on the research (2nd year master’s student).
(Interview and transcription: Chisato Hata)


