- Home
- New Kyurizukai
- Mechanistic Chemistry of Biomolecules
from Keio's Faculty of Science and Technology
Mechanistic Chemistry of Biomolecules
from Keio's Faculty of Science and Technology
The mechanism whereby metal ions
breathe life into proteins
Entering into the world of research, fascinated by the lifestyle of my respected mentors who enjoyed pure pursuit of sciences
So far Dr. Furukawa has met a number of wonderful people and enjoyed engaging in research in various places. He sincerely wishes that his students never forget the “joy of thinking” no matter how small or trivial the theme in question may seem. His experience of continuing research on metalloproteins, an interdisciplinary field between biochemistry and inorganic chemistry, makes him keenly aware of the importance of maintaining an interest in various fields of study.
Yoshiaki Furukawa
Born in Hyogo Prefecture, Dr. Furukawa specializes in bioinorganic chemistry, focusing on the roles metalloproteins play in physiological and pathological phenomena. He completed the doctorate course at Kyoto University Graduate School of Engineering and obtained a doctorate degree (Dr. Eng.). After serving Northwestern University of the United States and the RIKEN Brain Science Institute as a postdoctoral fellow, he joined Keio University Department of Chemistry as an associate professor (non-tenured) in April 2010, then assumed the current post in April 2015.
The Research
Associate Professor Yoshiaki Furukawa is featured in this issue, whose field of research focuses on mysteries of life phenomena through the metalloprotein formation process.
Binding of metal ions to proteins sustains our lives
Mechanistic chemistry of biomolecules sheds light on life phenomena on the molecular level
With the help of metal ions, proteins control numerous vital reactions. For example, a protein known as superoxide dismutase 1 (SOD1) begins to protect cells against reactive oxygen species only after it has bound copper and zinc ions. However, mutant forms of SOD1 incapable of metal binding form abnormal aggregates, which is considered to be a cause of amyotrophic lateral sclerosis (ALS). By focusing on an in vivo process that supplies metal ions to proteins, Dr. Yoshiaki Furukawa strives to shed light on various life phenomena and wishes to apply the results of his research to the prevention of incurable diseases and the development of remedies.
Metal ions indispensable to biological activities
Inside organisms, a variety of metal ions,such as iron,zinc, copper, molybdenum, cobalt, etc., exhibit their functions upon binding to proteins. Each and every one of these ions is indispensable to sustaining our lives. An environmental shift of tremendous magnitude that occurred on Earth in remote antiquity is believed to have much to do with the question why we humans came to need such a wide variety of metal ions.
Dr. Furukawa explains, “For example, hemoglobin, which is contained in our red blood cells, is a protein with an iron (II) ion. Hemoglobin can carry oxygen molecules because an oxygen molecule is bound to the iron (II) ion. Iron (II) ion is considered to be the first metal ion used by living things. This is presumably because this ion was abundant in oceans of the primeval Earth.” Proteins that have metal ions (hereinafter metalloproteins) caught Dr. Furukawa’s special interest, making him intent on studies of metalloprotein structures and functions.
Concentration of molecular oxygens in the atmosphere increased sharply as photosynthetic cyanobacteria appeared on Earth. As a result, most of the iron (II) ions were oxidized, which later accumulated on the sea bottom as insoluble iron(III) oxide. The use of iron ions suddenly limited, it is assumed that living things adapted themselves to environmental changes by smartly using copper and various other metal ions instead.
Protecting lives against reactive oxygen species by using metal ions
Of all proteins, about one third are said to bind some sort of metal ions. Why do living things need metal ions this much?
“Various chemical reactions are taking place inside organisms to sustain their lives. Characters behind these chemical reactions are proteins. Proteins are polymers consisting of amino acids, but proteins as such can produce only limited chemical reactions. By binding metal ions, however, the number of chemical reactions that proteins can afford increases in a single swoop.” Metal ions thus came to play vital roles indispensable to maintain highly advanced, sophisticated life phenomena.
The metalloprotein SOD1 (Cu/ Zn-superoxide dismutase) that Dr. Furukawa focuses on is an enzyme capable of removing the superoxide O2 - , a highly toxic reactive oxygen species. Malfunction of SOD1 increases the intracellular concentration of O2 - , which would damage DNA and/or membranes so much that living things won’t be able to live any longer. SOD1 consists of two subunits, each having a structure to which one each of a copper and zinc ion are bound (Fig. 1). The copper ion functions as the active site for removing O2 - while the zinc ion serves for stabilizing the SOD1 structure. Curiously enough, SOD1 can function within organisms by selectively binding copper and zinc ions only from among the diverse range of metal ions.
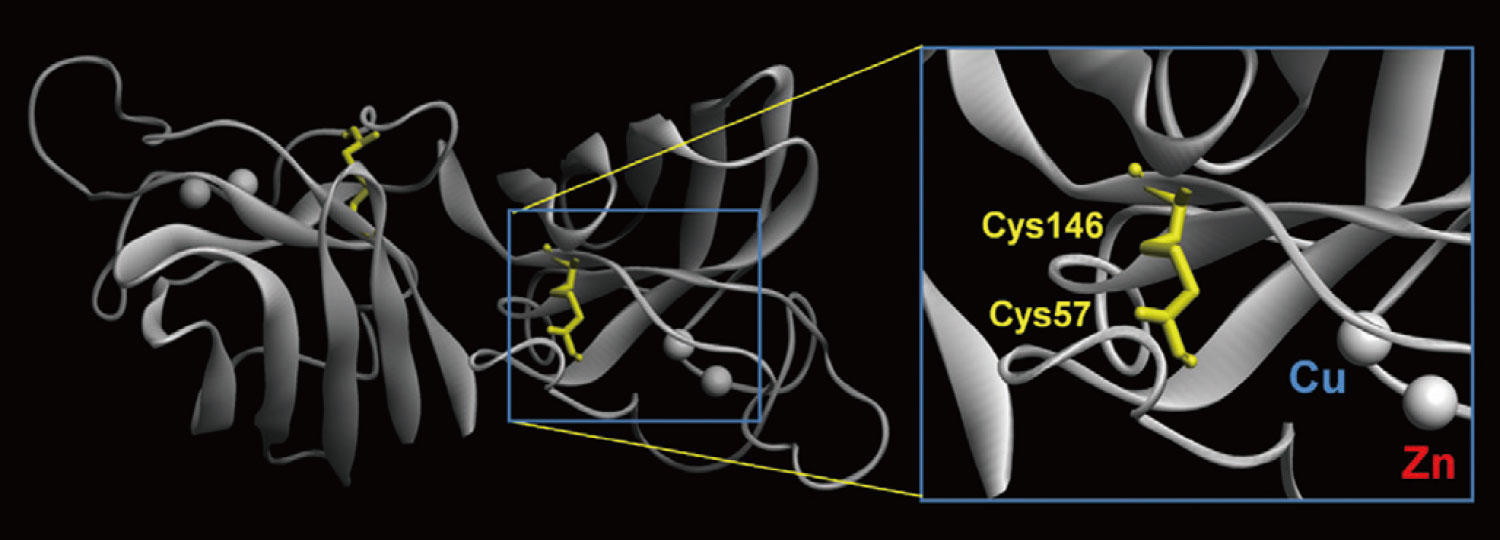
Fig.1 Structure of SOD1 protein
One each of copper (Cu) ion and zinc (Zn) ion is bound to each of the two subunits. Two cysteine residues (Cys57, Cys146) form a disulfide bond, which serves as something like a “lid” to prevent the Cu and Zn ions from getting dissociated.
How do proteins capture metal ions?
“Living things are unable to create metal ions themselves. Naturally, they have to take in metal ions from food. Since some metal ions are toxic, however, they need to capture specific metal ions only, bring them into their cells, and supply the metal ions to specific proteins. This is a very intriguing phenomenon, but much remains unknown about it,” says Dr. Furukawa, admitting that relationships between metal ions and proteins are still shrouded in mystery. It is likely that various biomolecules are scrambling for metal ions within cells, which leads to assumption of the existence of “metallochaperones” tasked with conveying metal ions to specific proteins. Prof. Valeria Culotta of Johns Hopkins University and Prof. Thomas O’Halloran of Northwestern University discovered copper chaperones for the first time in 1997. This accomplishment was followed by recent reports of discovery of iron and nickel chaperones. By the way, the chic-sounding term “chaperone” is a French word originally meaning a senior lady who teaches refined manners to young ladies about to make their debut in society.
For SOD1, a copper chaperone called CCS supplies copper ions. Dr. Furukawa found that SOD1 receives copper ions from CCS via the cycle shown in Fig. 2, and figured out that such copper ions protect the body against toxic reactive oxygen species. Furthermore, Dr. Furukawa determined that failure of this cycle hampers the supply of copper ions from CCS to SOD1. If that is the case, SOD1 cannot function as an enzyme and maintain its proper structure, causing numerous SOD1 molecules to aggregate – an abnormal phenomenon. Yet, Dr. Furukawa confesses he is still totally in the dark about how SOD1 captures a zinc ion. His challenge continues toward identifying the world’s first “zinc chaperone.”
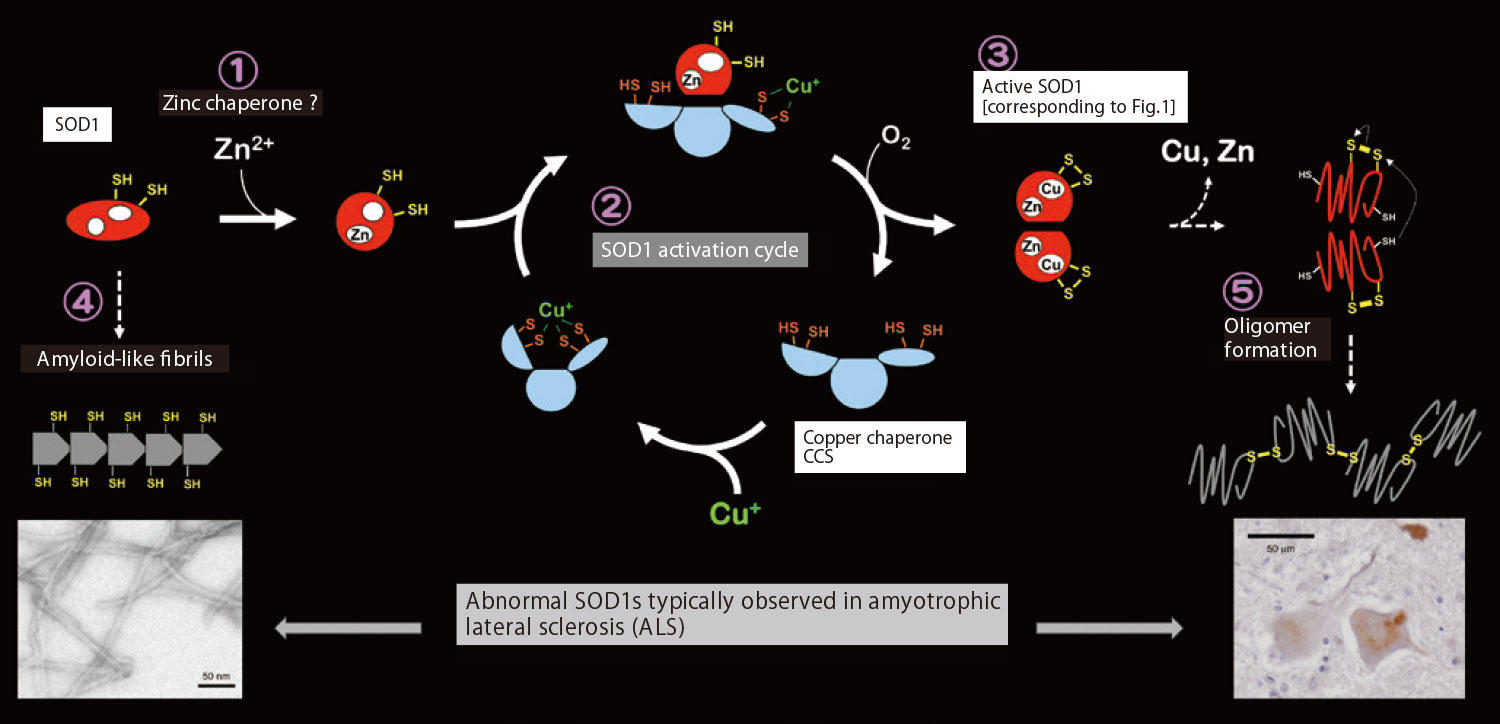
Fig.2 Dr. Furukawa-suggested mechanism of SOD1 activation and aggregation
① SOD1 binds a zinc ion first (mechanism unknown); ② then a copper ion is supplied by a copper chaperone, CCS; ③ SOD1 with enzymatic activity is produced as a result; metal-dissociated SOD1s aggregate into abnormal structures like ④ amyloids (photo: electron microscopic image) and ⑤ oligomers (photo: neurons of an ALS patient, the browncolored area being oligomer). Dr. Furukawa suggests that this mechanism concerns the development of ALS.
Failure of metal binding causes neurodegenerative diseases
“What I’d like to know is how proteins secure metal ions. The importance of this process can be clearly understood from the fact that proteins that failed to bind metal ions are observed in various diseases,” remarks Dr. Furukawa. While he asserts that his research is purely directed to basic studies, his research also attracts the attention of the medical circles given that SOD1 aggregates are a phenomenon observed in some amyotrophic lateral sclerosis (ALS) patients.
ALS is an incurable disease, with which nerves (motor neurons) that control body muscles are affected. This, in turn, causes muscles necessary for moving legs or breathing to atrophy. Many cases of hereditary ALS involve mutations in the SOD1-coding gene. As of now, more than 150 kinds of mutations have been reported in SOD1-coding gene.
What’s more, mutant SOD1s are found aggregating in motor neurons of the ALS patients. By controlling the binding of copper and zinc ions to SOD1, Dr. Furukawa succeeded in a test tube experiment to reproduce the process of SOD1 aggregation that develops within motor neurons of ALS patients.
“The question yet to be solved is whether SOD1 aggregation causes ALS or whether the development of ALS causes SOD1 to aggregate. This is the point we need to watch for. Even if we have successfully identified a substance that controls SOD1 aggregation, therefore, we cannot definitely say it will become an ALS remedy,” says Dr. Furukawa cautiously. Nevertheless, he continues to publish his own research results, convinced that clarifying the detailed mechanism of metal ion-controlled SOD1 activation and aggregation will eventually lead to a full understanding of ALS.
We may see the day come before long, when Dr. Furukawa’s genuine interest in metalloproteins will lead to the discovery of an innovative drug for this incurable disease.
Interview
Associate Professor Yoshiaki Furukawa
Awakening to the fun of learning as an elementary school student
What was your childhood like?
As an elementary schoolboy, I spent almost everyday playing baseball with my friends till dark immediately after coming back home from school. I wanted to become a professional star player at the Hanshin Tigers team in the future. I really meant it. I was also fond of insects and other living things. After school, I would go to a nearby open space to catch grasshoppers or to a rice paddy or irrigation canal to catch tadpoles and crayfish.
Although I was not so much interested in school studies, as an elementary school fifth or sixth grader, I was unexpectedly awakened to the “fun of thinking.” Mr. Goto in charge of our class at the time was a teacher of a somewhat peculiar type, who let us elementary school students think about the relationship between the brightness of stars and their distances to the Earth, or taught us about various properties of atoms using a periodic table of the elements. What’s more, he would often take us outdoors to let us understand the importance of observing and experiencing things in the field. Looking back at those days, I think these experiences might have been too advanced in content for us, but we could feel the fun of learning firsthand.
Why did you find an interest in chemistry?
During my junior and senior high school days, I took up challenges of the International Mathematical Olympiad and several other contests, where I found a world of enjoying the beauty of solving problems, rather than merely seeking to find answers. There I found bright people’s solving methods extremely simple. They did not try to rush at solving problems, but solved problems exquisitely after thinking them through. The enviable environment surrounded by such people, I think, led me to awaken to the fun of learning.
I still remember one day. During a chemistry class on the electronic theory of organic chemistry, I was fascinated by its clear-cut approaches. I was greatly motivated to study this simple, beautiful field – chemistry – more deeply and chose to learn at Kyoto University with a good reputation for chemistry.
At Kyoto University, however, I did not attend classes so diligently because the university in those days allowed its students to graduate only if they pass exams. So, I learned chemistry mainly by reading a variety of textbooks. Instead, I would actively take part in reading circles of Latin and German language seminars while also attending other seminars on the campus as an audit student. Thus I exposed myself to studies other than chemistry, which later proved to be very good experiences. By doing so, I was also able to make a number of good friends, with whom I fully enjoyed my campus life. In retrospect, however, it’s a pity I missed many of the classes by prominent professors representing Japan’s chemistry learning.
Choosing an interdisciplinary field between biochemistry and coordination chemistry
What motivated you to enter into the world of research?

The origin of my academic career is Prof. Isao Morishima’s lab, to which I was assigned as an undergrad. Back in those days, I already became interested in both biochemistry for molecular-level understanding of life phenomena and coordination chemistry that describes reactivity of molecules using molecular orbitals (MO’s). I was happy joining the Morishima lab and was able to satisfy both of my interests because the lab focused on the structure-function relationship of hemoproteins – an interdisciplinary area between proteins and metal complexes. In addition, the figure of Prof. Morishima that I saw when I visited the lab to look at it for the first time was so impressive I cannot forget it even today. Comfortably sitting on a large chair and smoking a pipe, he eagerly explained hemoglobin reactivity by the molecular orbitals of heme, which was truly inspiring.
Prof. Morishima was mentored by Prof. Kenichi Fukui who received Nobel Prize in Chemistry in 1981 for advocating HOMO/LUMO. To my great regret, I missed the opportunity to meet Prof. Fukui in person, but I often heard of this great scholar from Prof. Morishima, which was also a big driving force for me to enter into the world of research.
In the lab, I did research in electron transfer reaction of proteins while simultaneously learning biochemistry, spectroscopy and thermodynamics. Prof. Morishima was basically a man of leave-alone policy, so I did my research in my own way – testing and verifying what I thought I should do.
Despite some difficulties and distress experienced in the course of research, every day was really fulfilling – developing hot discussions with all lab members while writing this and that on white boards set up here and there within the lab. I’d also like to mention the then assistant professor Koichiro Ishimori, who thoroughly taught me how to communicate one’s idea to others, including how to write (not to mention scientific papers) and how to make a presentation at scientific meetings. I owe much to him for what I am today as a researcher.
What course of life did you have in mind after graduation?
So devoted to experiments, discussions and presentations, finding employment with a business was the very last thing I could think of. I suddenly found myself in the third year summer of the doctoral course without any plan for the future – a crisis situation. Thanks to recommendations from many professors, however, I was able to study under Prof. Thomas O’Halloran of Northwestern University who was active and world-renowned for his research in copper chaperone. I thought I might be able to shed some light on life phenomena and the development of various diseases if, in the course of my research in copper chaperone, I could clarify interactions between proteins and metal ions. This idea continues to be the source of energy behind my current research activities.
“Everything should be as simple as possible, but not simpler.” Prof. O’Halloran often cited this remark of Albert Einstein. While Prof. O’Halloran is currently engaged also in other projects, I’m overwhelmed by his ability to come up with one innovative idea after another. He advised me, saying “Don’t be afraid of creating new ideas, but just enjoy it.” Thanks to this encouraging advice, I’m coming to enjoy my research work even more.
Later, I joined Dr. Nobuyuki Nukina’s research team at RIKEN Brain Science Institute in Japan. Dr. Nukina was one of the few neurologists, who were attempting to understand the pathologies of neurodegenerative diseases by focusing on structural changes in proteins. Even today, he sticks to basic studies. As a member of his team, I was privileged to freely use cultured cells and experimental models like mice and rats I had never handled before, as well as costly experimental equipment. These experiences helped me a lot in honing my experimental skills. In this sense, I may call it the most fulfilling period of my life.
In retrospect, I have been truly blessed with good mentors. Each and every one of my mentors was enjoying their scientific pursuits and their own lives, thus providing me with good role models for my life.
Universities are important venues where you, students, should think about your future paths.
What do you expect of your current students?
I joined Keio University in 2010. Fortunately, I was able to set up my own lab soon thanks to enthusiastic support of Department of Chemistry professors who voiced, “Keio needs to introduce a new field of research.”
With alumni of many graduates who are active at the forefront of society, I feel Keio University is a driving force of Japan, so to speak. On one hand, it is wonderful. On the other hand, I’d advise our students to not become too dependent on the Keio brand and its extensive human network. I’d like my students to develop and exhibit their own individual colors. I’d also like to see Keio demonstrate its advantages as a private educational institution not easily affected by national policies.
Another point I’d like to make is we should stop distinguishing the fields of learning into chemistry, physics, biology, etc., or worrying much about distinguishing between basics and application. I say this because I think this kind of idea may eventually hamper the understanding of sciences, given learning originally has no demarcations.
Universities are where you should think about your future paths, not a mere waypoint to find employment with big businesses. Prof. Morishima once taught me the phrase “noblesse oblige.” This means the noble have their obligations to perform for society. Our students are privileged to enjoy such an enviable environment as Keio. Therefore, they should think more seriously about what they should do to contribute to society, and live their lives with pride.
How do you refresh yourself when you have time to spare from research?

I like languages and letters/characters of the world, so I often take my family to exhibitions and events that interest me. The catalyst for my liking were the ancient Tangut characters that I knew in the novel “Dunhuang” authored by Yasushi Inoue that I read when I was a junior (or senior ?) high school student. As introduced in the novel, Tangut characters appear to be Chinese characters but they are actually not. Then what are these characters really like? I remember I was obsessed by this question. Since the Internet was not in use in those days, I visited libraries and bookstores here and there trying to find out what the ancient characters really are. I still remember I was struck with a strange feeling beyond description when I first saw Tangut characters. With this experience as a start, I came to know there are many different characters around the world. Indeed, simply looking at little known foreign characters soothes me.
Becoming so dissatisfied with merely looking at books, I wanted to see such characters firsthand, which drove me to travel abroad. In fact, I visited numerous foreign destinations just to look for ancient characters – such as Egypt (for hieroglyph), Iran (cuneiform characters), Mexico (Mayan script), among others. In China, I visited as far as the city of Kashgar (Xinjian-Uygur Autonomous Region), where the Uighur language is written using Chinese characters. I enjoyed looking at a flood of seemingly Chinese characters that I totally couldn’t understand. It was a truly stimulating experience. By nature, I like traveling. This is mainly because my parents would often take me overseas from the time when I was a child. Maybe because of this background, I still like to travel overseas with persons with whom I can share joy and distress – to see intriguing characters of the world. In fact, I visited various overseas destinations, sometimes involving my wife even before our marriage.
As for daily breathers, the best is playing with my daughters, an elementary school girl and a kindergarten student. Simply looking at them is really fun!
Some words from students
Student : Dr. Furukawa came to Keio in 2010. It just happened to be when I entered Keio. Eager to challenge a research theme dealing with substances like proteins with large molecular weight, I jumped at the Furukawa lab engaged in research in metalloproteins. I’m now in the first year of the doctoral course. Dr. Furukawa puts faith in me and allows me to carry out research as freely as I like, which is encouraging as well as comfortable.
(Reporter & text writer: Akiko Ikeda)


