- Home
- New Kyurizukai
- The Science of Electrodes from Keio's Faculty of Science and Technology
The Science of Electrodes from Keio's Faculty of Science and Technology
Being thorough and carving a path within a school that values independence
Yamamoto describes himself as unable to move on from something until he is convinced that he has examined it from every possible angle. Along the way, he is able to give his all to issues that demand his undivided attention. One can only imagine how this painstakingly accumulated expertise and experience might be used to fuel future research and education at the university.
Takashi Yamamoto
Specializes in electro-organic synthesis and solid-state chemistry. Yamamoto graduated with a bachelor’s degree from Keio University’s Department of Chemistry in the Faculty of Science and Technology in 2002. He then went on to complete a doctoral program at the Graduate School of Science and Technology in 2007, receiving his Ph.D. in Science. He began working at Keio University after completing positions as a postdoctoral research fellow with the Japan Society for the Promotion of Science (JSPS), a visiting research fellow at the University of Florida, and an industry-academia-government project researcher with the Tokyo Institute of Technology’s Laboratory for Chemistry and Life Science . At Keio, he was hired as a non-tenured assistant professor with the Faculty of Science and Technology in 2009, was promoted to assistant professor in 2011, became a senior assistant professor in 2014, and assumed his current role as an associate professor in 2023. From 2016 to 2017, he also worked concurrently as a visiting research fellow at the University of Mainz.
The Research
This feature explores how Associate Professor Takashi Yamamoto is using “electro-organic synthesis” to find green and sustainable ways for manufacturing processes..
Electrifying Organic Synthesis
Synthesizing organic compounds using electricity, known as “electro-organic synthesis,” has received a world-wide attention for its potential in reducing CO2 emissions and reagent wastes, thereby realizing a more sustainable manufacturing process. In this increasingly competitive field, Associate Professor Yamamoto hopes to replace conventional manufacturing with new methods through his own unique approach.
Organic compounds essential for our daily life
We are surrounded by man-made objects. Among them, organic compounds (carbon-based substances) play a vital role in our daily life, as plastics, elastics, synthetic fibres, and pharmaceuticals.
Synthesis of organic compounds generically requires “special reagents”, e.g. catalysts, to accelerate chemical reactions. In addition, huge amounts of energy are often required because some reactions take place at high temperature. Of course, while most reagent wastes produced from the reactions are properly collected and disposed of, the environmental impact could be significantly reduced by excluding such reagent wastes from the process.
This is the background of “electro-organic synthesis”. Associate Professor Yamamoto at the Department of Chemistry is one of the leading researchers in this exciting field.
Electro-organic synthesis?
Do you remember learning about electrolysis in junior high school? When an anode and cathode are immersed in water (H2O) and electricity is applied , hydrogen (H2) is generated at the cathode and oxygen (O2) is generated at the anode. “Electro-organic synthesis” applys such electron transfer events to create new organic compounds from other organic compounds (Figure 1). A chemical reaction is driven by electricity instead of special reagents.
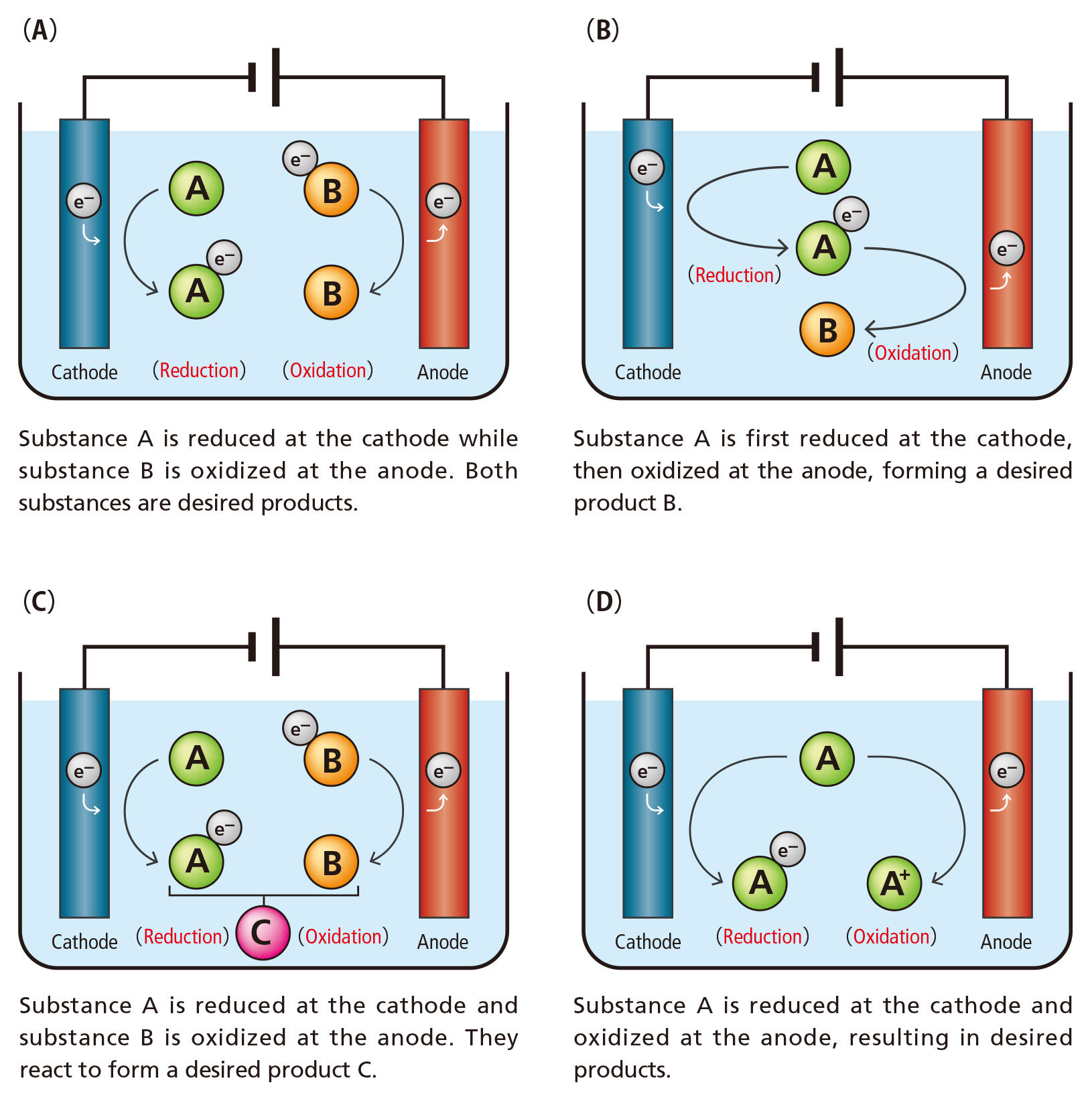
Figure 1: Paired electrosynthesis for more efficient use of electricity Reduction (receiving electrons) and oxidation (losing electrons) occurs at the cathode and anode, respectively. Activated species by this process react to produce a desired substance. Even though electro-organic synthesis does not use special reagents, energy is wasted if the electricity is not used efficiently. In order to avoid this problem, researchers in electro-organic synthesis have been developing "paired electrosynthesis," which leads to the synthesis of target substances at both the cathode and the anode.
Main achievement during a short-term study at the University of Mainz
Yamamoto started researching electro-organic synthesis in 2016, when he stayed at Johannes Gutenberg University Mainz (University of Mainz) in Germany. “At that time, I was struggling to keep up with the speed of research field that I had been working in, so I was feeling quite anxious about my future. Fortunately, I was given the opportunity to study abroad through the Keio University Ishii-Ishibashi Fund for Young Researchers. At the time, I had been working as a senior assistant professor with Professor Yasuaki Einaga. I wanted to try breaking new ground that can be continued when I returned to Japan. Since Professor Einaga is working on the chemistry of diamond electrodes, I searched a research topic related to the diamond electrodes.” Thus, I began research on electro-organic synthesis using diamond electrodes with Professor Siegfried R. Waldvogel at the University of Mainz.
In 2017, he succeeded in synthesizing α-diisoeugenol from isoeugenol (Figure 2). “Even though the starting material was the same, when solvent was changed and applied an electric current, the products were different from what I expected. Even though I had been studying chemistry for a long time, I was so shocked that such a thing happens.”
This is not the only thing to shock Yamamoto. In chemical synthesis, stereoisomers—isomeric molecules that have the same chemical formula, but different spatial arrangements of their atoms—are obtained mixed together without any special trick. Because stereoisomers have different properties, it is necessary to separate them once mixed. Therefore, one of the major challenges in chemical synthesis lies in easily produce only the stereoisomer of interst. To return to Yamamoto’s experiment,only α-diisoeugenol was produced and no other stereoisomers were present.
Based on what he learned and achieved in Germany, Yamamoto is now working electro-organic synthesis using diamond electrodes at the Einaga Group.
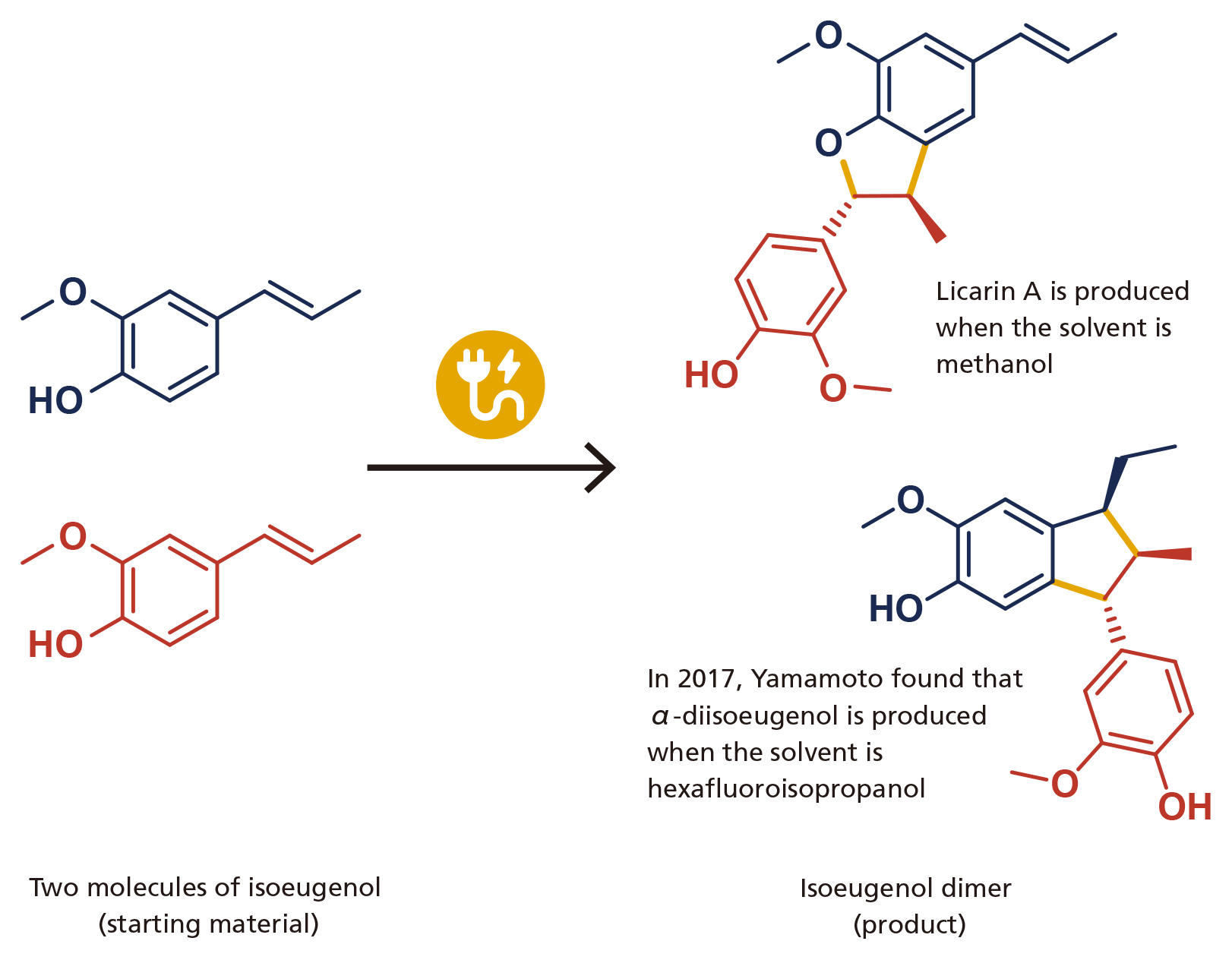
図2 Electro-organic synthesis using isoeugenol as a starting material This is the main achievement that Yamamoto had done in University of Mainz. When hexafluoroisopropanol was used as a solvent, α-diisoeugenol was formed from two isoeugenol molecules (bottom). Previously, it was known that licarin A was formed when methanol was used as a solvent (top). It was a big surprise that changing the solvent had a strong impact on the product. Yellow lines indicate newly formed bonds.
Intense competition in green and sustainable manufacturing
The situation has changed since Yamamoto has started researchin electro-organic synthesis,. “Attempts to synthesize substances using electricity have a long history, dating back to the 1800s. In the late 1900s, the field of electro-organic synthesis made great progress, but the number of researchers working in this field did not increase significantly because of the lack of equipment that anyone could easily handle. Now, however, the situation is completely different. Various companies sell electrosynthesis equipment that is easy to use, lowering barriers to starting research in electro-organic synthesis. Electro-organic synthesis not only avoids the use of special reagents, but also allows power to be supplied from renewable energy sources such as solar power and wind. Therefore, electro-organic synthesis is undergoing a worldwide development race, as enables manufacturing that can reduce CO2 emissions.”
Competing with the world through research that only I can do
However, Yamamoto is not anxious about the future of his research as he used to be. This is because he is absolutely confident in the electrode material that is a significantly important factor in electro-organic synthesis.
“The diamond electrodes prepared at the Einaga Group have several excellent properties for electro-organic synthesis that other electrodes do not have. I will explain two representatives. First, the highly reactive radical species that are produced by applying electric current are stable at the surface of diamond electrodes. Second, diamond electrodes can be used over a wide range of voltages. Overall, diamond electrodes enable to realize chemical reactions that would not be possible with commonly used electrodes.”
Yamamoto is also attempting to make own improvements to their diamond electrodes. “ My previous research project was developing the optical and magnetic properties of inorganic nanosheets towards next-generation devices. I can make a modification of the electrode surface based on the experiences and techniques I acquired from those studies. It is really interesting to see how changing the properties of electrode surface influences on a yield and selectivity of products. I aim to pioneer a new ‘science of electrodes’ by establishing a fundamental theory behind these phenomena.”
Yamamoto also talked about the various things he aims to accomplish. His ultimate goal is to “realize chemical reactions in electro-organic chemistry that even high school students can understand.” “An example would be to replace the reactions that are already in textbooks and used in industry (e.g. reactions that require high temperatures and pressures) with electro-organic synthesis. Realizing this goal would be the only way I can say that I have achieved green and sustainable manufacturing.”
Electro-organic synthesis is currently facing a wave of intense competition. Yamamoto is drawing on his breadth of experiences and making strong strides forward to achieve his impressive goals as a researcher.
Interview
The Interview: Associate Professor Takashi Yamamoto
Discovering the difficulty and delight of chemistry as a college student
I understand that your father also worked as a professor at a university.
That’s true. He specialized in soil microbiology and taught at Shimane University. As a child, I would often visit my father’s lab, which made me want to follow in his footsteps by studying agriculture. As fate would have it, I actually failed the exams for the national university I applied to for agricultural studies. As a result, I ended up going to Keio University. That’s how I got my start in chemistry.
One ironic story I like to share from that time was how my older brother accompanied me on the bullet train when I was visiting Tokyo to finish up some admissions’ paperwork for Keio. He looked out the window and shouted, “You can see the Pen Mark logo!” I still remember how I felt gazing up dejectedly at the Pen Mark on one of the Yagami Campus buildings.
How was your time at Keio as a student?
I was surprised by the gap between what I had studied of chemistry in high school compared to what they taught in college. First off, I had never learned about quantum chemistry in high school, so I didn’t even know that it even counted as chemistry! I was especially worried that I would fail organic chemistry. If you don’t study, you don’t know how electrons move, and if you don’t know how electrons move, you don’t know how they’ll react. I had my organic chemistry classes on Fridays. Afterwards, I would go get dinner somewhere in Hiyoura (the shopping district on the opposite side of Hiyoshi station from the university), head back to the university by 7 p.m., and study for around two hours at the Media Center (the library on Hiyoshi Campus) until it closed. I was in a choir back in high school, but since it was such a surprisingly demanding hobby—you had to be careful about taking care of your throat and had to work out a lot—I quit as soon as I started college. I wasn’t really involved with extracurriculars as an undergrad. I had enough on my plate living by myself for the first time in a completely new city. Otherwise, I did the odd job working as staff for events or as a private tutor.
How did your working relationship with Professor Yasuaki Einaga develop when you joined his laboratory as a fourth-year student?

I was in the first-ever cohort for the Einaga Laboratory. He was still relatively early in his career, and we had a lot of free time to get to know each other in the lab. It has made it easy to continue working on research together. The ways that we approach our research, though, are quite different. He’s what I would call an “elevator type,” someone who has a goal and moves linearly towards that goal. I am more of a “staircase type.” I like to be sure of my footing and test what I can do every step of the way. I think these differences have actually contributed greatly to our success in working together. But I remember back when I had just started out in the lab as a fourth-year student that I would sometimes spend up to a week holed up in the library looking up different references and research studies in order to satisfy Professor Einaga with my experiment results. Because I am so thorough and don’t like to move on without covering all of my bases, my current students seem to think of me as being incredibly detail-oriented [laughs].
“Training montage” and becoming a Keio faculty member
Can you tell us more about your experiences studying abroad and working at other universities?
I studied abroad in 2007 at Florida University to learn from Professor Daniel Talham. I admired how detailed his work was and I had always been interested in the types of research he was doing. In person, he was someone who really valued language. He was the one who taught me what it means to explain something accurately.
After returning to Japan in May of 2008, I studied polymers at the Tokyo Institute of Technology (renamed October 2024 to “Institute of Science Tokyo”) under Professor Tomokazu Iyoda. He was incredibly strict. Maybe it was because I never met his expectations of how much research I should be completing as a project researcher, or maybe it was because he wanted me to model what it meant to be a researcher for my underclassmen. Either way, I couldn’t escape him even in my dreams [laughs]. However, I can’t deny that those experiences shaped who I am now as a researcher. I also learned that for every breakthrough research paper or shiny-new technology, there are innumerable mundane and rough experiments taking place behind the scenes. The amount of research I worked on while there wasn’t half bad, but because I was also asked to join in the inter-laboratory volleyball tournament at the Suzukakedai campus, it was a tough year physically as well as mentally.
Fast-forwarding to April 2009, what was it like to return to Keio University?
When I told my family that I would be starting as a faculty member in April, the first thing my father said was, “Don’t forget that you are an educator before you are a researcher.” I was shocked. It was the first time I had to come to terms with the fact that I would be teaching, despite the fact that I had spent every waking hour until then focused exclusively on my research and experiments. He also introduced Tsuneo Sasaki’s Words of Advice for Young Working People (as described in “My Favorite Books”).
I don’t know if it was because I had left for a while, but returning to Keio University felt like coming home. It was a very comfortable transition. However, working as a faculty member and being responsible for teaching courses was an entirely new challenge. Because I had been steeped in various “Iyoda-isms,” I think I was quite strict with my students at first. But my strictness in seminars (rinkou) is so that I can ascertain whether my students have fully understood the most important points in the material we cover.
I am always trying my best to be able to answer my students’ questions as thoroughly as possible. Keio students are all incredibly bright and talented. They’ll often look things up on their own before coming to me. In a way, I am the “last line of defense.” If my students come to me, I want to make sure that I am approachable and a presence that can offer them advice and hints about what to do to make progress. This is what inspires me to keep learning every day and it is my personal interpretation of the phrase here at Keio, “learning while teaching, teaching while learning.”
After deciding to head in a new direction
I have heard that you made a drastic change in your research in 2016. What inspired that decision?

I felt like I had become stagnant in my research, and when I looked at the amount of time I had left in my career as a researcher, I figured I still had time to pivot to a new field (as seen in my research overview). I had lived in the United States when I was an elementary student due to my father’s job, and I had also experienced time abroad in 2007 at Florida University. I had always wanted to study abroad in Europe someday, and I was fortunate enough to be a research fellow at a university in Germany. However, I still had my responsibilities here at Keio University as a faculty member, so it was a study abroad experience limited to the spring and summer breaks. The laboratory where I studied (at Johannes Gutenberg University Mainz) was led by renowned scientist Dr. Waldvogel. There were many excellent students at the lab at the time and the program was designed to allow international students to participate easily. It was an “iron sharpens iron” environment, and I am very proud of the research results we produced (as seen in my research overview). I also learned more about how to conduct research. At the lab, most students would arrive around 8:30 a.m. with experiments lasting from 9:00 a.m. until 11:30 a.m. Everyone would take a break for lunch, attend a mid-day meeting, and then work on experiments again from 1:00 p.m. until 3:00 p.m. We would then have another quick snack break, return to work at 3:30 p.m., and finish experiments by 5:00 p.m.. Most people had finished tidying up and left the lab by 5:30 p.m. Despite this compact schedule, we were constantly producing results that could be published in top-tier scientific journals. This made me realize firsthand that long hours in a laboratory are not always the “right” answer. The important thing is to be able to focus and give your full attention to the research at hand.
What would you like to say to any high schoolers who plan to enroll at Keio or to the college students already here?
I have been learning flower arrangement from Nicolai Bergmann at his flagship store in Aoyama since May 2010. For my research, I had studied with Professor Talham and Professor Waldvogel, both of whom are first class in their areas of expertise. If you want to be first class in any given specialty, you need to encounter things that are “first class.” Eat “first-class” food, observe “first-class” art, come into contact with “first-class” items. Working on floral arrangements is a great palate cleanser for me, because it lets me forget myself in the beauty of the flowers I handle.
I personally think that Keio University is an incredibly liberating environment compared to other schools partly because of its commitment to Yukichi Fukuzawa’s teachings about “independence and self-respect.” Both students and faculty value each other's autonomy. In return, individuals have to practice self-discipline, but I think it’s incredibly valuable that it is an environment in which people are challenged, not reproached for failing those challenges, and able to receive critiques on their processes. It’s an environment that promotes growth in both students and faculty members, allowing us all to bloom at our own pace.
Some words from Students…
●Professor Yamamoto has an amazing sense of humor and always enjoys talking with his students. I was working on some basic research about how to change an electrode’s properties by altering the functional groups of a diamond electrode’s surface. You have to use a lot of different equipment in order to be able to observe electrode surfaces, which can take a while to master. Professor Yamamoto is so knowledgeable about this type of thing that he knows how to give the perfect advice. His motto is “Never be aimless while conducting an experiment.” There is a lot of repetition for our research, but I’ve learned to remain conscious of the reasons behind what I am doing while I am working. Professor Yamamoto is also wonderful about providing feedback on the reports we submit each week, pointing out grammatical errors or faulty logic. As a researcher, I feel like I am able to learn a lot from him as my professor (First-year master’s student).
(Interview transcription and composition: Akiko Ikeda)


